1/ The significant importance of studying locomotion and muscle physiology in zebrafish
Zebrafish are widely recognized as a valuable model organism in biological research due to their genetic similarity to humans, rapid development, and transparent embryos. These characteristics make them ideal for studying various biological processes, including muscle development and function.
Zebrafish also provide crucial insights into the genetic basis of muscle diseases and disorders. Their well-characterized genome and the ease of genetic manipulation allow researchers to create models of human muscle diseases, such as muscular dystrophy, and study the underlying genetic mechanisms.
The transparent embryos of zebrafish enable real-time observation of muscle development and growth. This helps researchers understand the fundamental processes of myogenesis and the formation of the musculoskeletal system.
Zebrafish are used to model a wide range of human diseases, including metabolic disorders and age-related muscle degeneration. Studying locomotion and muscle physiology in these models can reveal potential therapeutic targets and interventions.
The use of zebrafish in high-throughput screening assays facilitates the discovery of new drugs and compounds that can modulate muscle function and treat muscle-related diseases. Their small size and rapid reproduction make them cost-effective for large-scale studies.
Locomotion is a critical behavior that reflects the overall health and physiological state of an organism. By studying locomotion in zebrafish, researchers can gain insights into the functional outcomes of genetic modifications, environmental factors, and disease conditions.
Muscle physiology is closely linked to metabolism. Zebrafish provide a useful model for investigating the metabolic pathways involved in muscle function and how they are regulated during exercise and other physiological states.
Zebrafish have a remarkable capacity for muscle regeneration. Studying the mechanisms underlying this regenerative ability can inform research into regenerative medicine and tissue repair in humans.
2/ "Protocol to measure spontaneous locomotion, graded exercise oxygen consumption, and cross-sectional area of skeletal muscle cells in zebrafish" -
Mauricio Castro-Sepulveda 1 , Cassandra Tabasso 1 , Dogan Grepper 1, Adrien Martinotti 1, Axel K.F. Aguettaz 1, Sylviane Lagarrigue 1, Francesca Amati 1 2
1 Aging and Muscle Metabolism Lab, Department of Biomedical Sciences, Faculty of Biology and Medicine, University of Lausanne, Bugnon 7, Lausanne, 1005 Vaud, Switzerland
2 Service of Endocrinology, Diabetes and Metabolism, Lausanne University Hospital and University of Lausanne, Lausanne, 1011 Vaud, Switzerland
In this publication, Almati's Lab presents a comprehensive protocol for skeletal muscle phenotyping in zebrafish, which serves as a valuable model organism for studying muscle function and metabolic health. The protocol developed by Francesca Almati’s lab includes detailed steps to evaluate spontaneous locomotion, measure oxygen consumption during incremental exercise, and analyze the cross-sectional area of skeletal muscle cells in adult zebrafish :
-
Spontaneous Locomotion: The protocol involves assessing the spontaneous movement of zebrafish, which is a critical indicator of muscle function and overall health. This measurement helps in understanding the baseline activity levels and can be used to detect any abnormalities or effects of genetic modifications and environmental factors.
-
Graded Exercise Oxygen Consumption: The method includes measuring oxygen consumption during graded exercise. This is done to evaluate the metabolic response of zebrafish to increasing levels of physical activity. By monitoring oxygen consumption, researchers can infer the efficiency of muscle metabolism and overall cardiovascular health.
-
Cross-Sectional Area of Skeletal Muscle Cells: The protocol also describes techniques to analyze the cross-sectional area of skeletal muscle cells. This involves histological methods to prepare and examine muscle tissue samples. Changes in muscle cell size can indicate muscle growth or atrophy and provide insights into the physiological adaptations to exercise or disease conditions.
The significance of this protocol lies in its potential applications for assessing muscle quality and function in various research contexts, including studies on metabolic diseases, aging, and skeletal muscle health. The use of zebrafish as a model organism offers several advantages, such as their genetic similarity to humans, rapid development, and the ability to conduct high-throughput screening.
Francesca Almati’s work emphasizes the importance of these measurements in providing a comprehensive understanding of muscle physiology and the impact of different interventions on muscle health. The detailed protocol allows for standardized and reproducible assessments, making it a valuable tool for researchers in the field of muscle biology and metabolic health.
See the publication here https://www.sciencedirect.com/science/article/pii/S2666166725002692
3/ How to measure locomotion in zebrafish ?
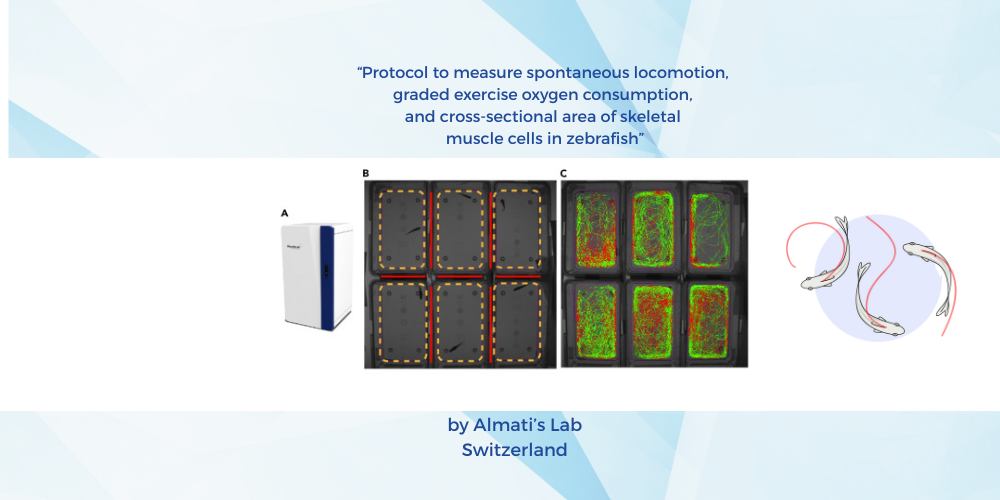
ZebraBox and ZebraCube are innovative tools for measuring locomotion
In the study conducted by Francesca Almati’s lab, the use of advanced tools such as ZebraBox and ZebraCube has revolutionized the way researchers measure locomotion in zebrafish. These tools provide precise and reliable data, enabling scientists to gain deeper insights into the behavioral and physiological aspects of zebrafish.
ZebraBox is a sophisticated system designed to track the spontaneous locomotion of zebrafish larvae. This tool utilizes high-resolution cameras and advanced software algorithms to monitor the movement patterns of larvae in a controlled environment. By analyzing the data collected, researchers can assess various parameters such as distance traveled, velocity, and activity levels. This information is crucial for understanding the developmental and behavioral aspects of zebrafish larvae, as well as for identifying any abnormalities or changes in locomotion due to genetic modifications or environmental factors.
One of the key advantages of using ZebraBox is its ability to provide continuous and automated monitoring. This eliminates the need for manual observations, which can be time-consuming and prone to human error. Additionally, ZebraBox allows for the simultaneous monitoring of multiple larvae, increasing the efficiency and throughput of experiments. This high-throughput capability is particularly beneficial for large-scale studies and screening assays, where the ability to process large amounts of data quickly is essential.
On the other hand, ZebraCube is specifically designed for measuring locomotion in adult zebrafish. This tool offers a more spacious and adaptable environment, allowing adult zebrafish to exhibit their natural swimming behaviors. ZebraCube is equipped with advanced tracking software that can accurately measure various locomotion parameters, including swimming speed, distance traveled, and turning angles. This data is invaluable for studying the effects of aging, disease, and environmental factors on the locomotion of adult zebrafish.
The use of ZebraCube also provides several advantages over traditional methods. For instance, it allows for the non-invasive monitoring of zebrafish, reducing stress and potential interference with their natural behaviors. Furthermore, ZebraCube can be easily integrated with other experimental setups, such as those used for measuring oxygen consumption or muscle physiology. This integration enables researchers to obtain a comprehensive understanding of the relationship between locomotion and other physiological parameters.
In conclusion, the use of ZebraBox and ZebraCube in Francesca Almati’s lab has significantly advanced our understanding of locomotion in zebrafish. These innovative tools provide precise, reliable, and high-throughput data, enabling researchers to gain deeper insights into the behavioral and physiological aspects of zebrafish. The findings from this study have important implications for the broader scientific community and highlight the potential of these tools for future research in this area.