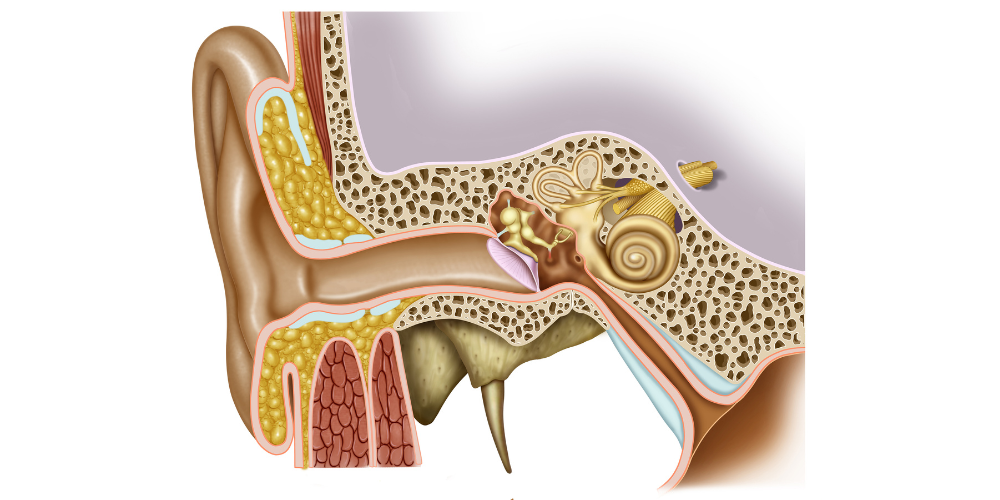
In humans, the ear protects two systems: auditory and vestibular; these are responsible, respectively, of two senses: hearing and equilibrium. These senses are extremely interesting to study among all the things we can research about the human body.
Their structure allows for the detection of mechanical stimuli and the interpretation of these stimuli as sounds, gravity or acceleration, depending on the location of the detection. This transformation is possible due to a shared sensory cell: the hair cell.
The particularity of these cells, which is responsible for their name, is the presence of hair-like extensions that protrude into the fluid surrounding them. The shorter of these hair-like extensions are the stereocilia, and they are arranged in a staircase-like pattern next to a single large cilium known as a kinocilium.
All cilia are strongly linked together by proteins. Thanks to this, when the kinocilium moves, it makes all the stereocilia move as a single unit, called the hair bundle. In sum, when the surrounding fluid moves, it makes the kinocilium move and, consequently, the hair bundle with it.
The movement of the hair bundle forces the opening of some channels placed at the tip of the stereocilia (tip links). Upon their opening, the channels pass current and generate an electrical output to the brain, which is then interpreted as sounds, gravity or acceleration. The conversion of a mechanical stimulus into an electrical signal is known as mechano-electrical transduction (MET).
The mechanism behind mechano-electrical transduction
The proteins responsible for the MET are structured in a large protein complex. A handful of members of this MET complex have been identified, but we do not fully understand how the proteins interact. It is also unknown how the MET complex stays fixed at the stereocilia tips.
Several studies have revealed candidates for the main MET complex proteins: the Transmembrane Channel-like (TMC) proteins TMC1 and TMC2. In zebrafish, their equivalents are Tmc2a and Tmc2b. They are responsible for MET in hair cells of the lateral line organ, an organ specific to fish and amphibians.
Another key component of the complex is the transmembrane inner ear (TMIE) protein.
Zebrafish lacking the gene that produces Tmie (ru1000 mutants) lose sensitivity for mechanical stimuli. Moreover, if Tmie is restituted they regain sensitivity. An interesting effect that was observed during the experiments with ru1000 mutants was that, on top of losing sensitivity for mechanical stimuli, Tmc1 and Tmc2b were absent of the hair bundle. And, in the opposite direction, if Tmie levels were increased, this led to an increase in Tmc1 and Tmc2b in the hair bundle.
Evidence suggests that Tmie is important for Tmc1 and Tmc2b presence in the hair bundle. However, details of the relationship are unknown. Thus, Pacentine and Nicolson, in the present study, aimed at unraveling this relationship. They analyzed Tmie ru1000 mutants (absent Tmie) and observed the result of introducing Tmie in them.
The team first analyzed the morphology in Tmie ru1000 mutants, which was normal. Hair cells, found in the inner ear and the lateral line organ, which is an organ specific to fish and amphibians, were also normal.
ZebraBox monitors auditory response
After morphological evaluation, they evaluated hearing capacity. This was possible with the auditory evoked behavioral response (AEBR) test. For the test, larvae were placed in six central wells of a 96-well plate mounted on an audio speaker. Tones were played every 15 seconds for 3 minutes. Movement responses to the tones were recorded in the dark inside a ZebraBox monitoring system, which automates the recording procedure, making the evaluation much easier. The response was recorded as positive if it occurred within two seconds after the tone and surpassed a threshold.
The ZebraBox system is perfect for behavioral research with zebrafish. It offers controlled experimental conditions and reliable results for up to 96 individuals at the same time (even more if multiple ZebraBox apparatus are used at once). ZebraBox provides long-term monitoring without compromising experimental conditions thanks to its integrated illumination, including infrared system, and water flow system. Moreover, it is a soundproof system, which makes it optimal for tests like AEBR.

Tmie-absent zebrafish are deaf
The results of the AEBR test indicated that Tmie-deficient zebrafish are deaf due to a defect in hair-cell sensitivity to mechanical stimuli.
After this evaluation, the team proceeded to reintroduce Tmie in Tmie ru1000 mutants. With this procedure, they discovered that Tmie can traffic into the MET complex without any other members of the complex. However, Tmc1 and Tmc2b cannot localize in the stereocilia without Tmie. When Tmie’ presence increases, Tmc1 and Tmc2b also increase in the hair bundle.
The ability of Tmie to traffic on its own to the hair bundle is due to a specific region of the protein, which accounts for this property. Parallel to this, another specific region of the protein is responsible for normal function of the hair cell. These same regions that mediate hair-cell function are responsible for localizing Tmc2b in the hair bundle.
The importance and complexity of Tmie
In sum, the results of the team demonstrate that Tmc1 and 2b require Tmie for trafficking to the hair bundle, while other known members of the MET complex can traffic normally in Tmie mutants. These findings suggest, thus, that Tmie-Tmcs traffic together in one unit, while other members go in a separate unit. This indicates that the MET complex proteins traffic to stereocilia in separate groups and then assemble at the hair bundle.
The relationship between Tmie and Tmc1/2b is very strong. When Tmie is absent from the bundles, so are Tmc 1 and 2b; when Tmie is increased, the bundle levels of Tmc1 and 2b are increased as well.
Moreover, Tmie has different regions required for localization and function, which indicates that Tmie’s functional role is separated from its ability to traffic to the hair bundle.
The precise mechanism underlying Tmie’s regulation of the Tmcs needs further investigation and will be researched in future studies, aiming at unraveling the molecular mechanisms that lead to hearing and equilibrium.
References
Pacentine IV, Nicolson T. (2019). Subunits of the mechano-electrical transduction channel, Tmc1/2b, require Tmie to localize in zebrafish sensory hair cells. PLoS Genet. 15(2): e1007635. https://doi.org/10.1371/journal.pgen.1007635
Related products
ZebraBox, VideoTrack and ZebraLab